If some salt is added to the water, it will dissociate into positive and negative ions. Negative ions will gather around positively charged colloidal particles while positive ions will be repelled. This way the electric field of the colloidal particle will be screened. If enough salt is added, the colloid will precipitate out. (This is called salting out a colloid.)
Find the electric potential around a colloidal particle! Consider the concentration of positive and negative ions to be the same far away from the particle. You will need the formula for the Boltzmann-distribution: particles in a potential are distributed in such a way that the number of particles having potential energy is proportional to . For this calculation consider the case when .
|
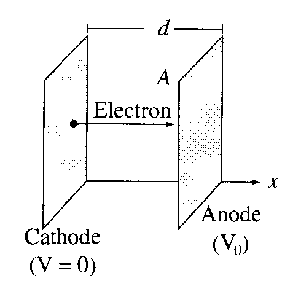
Suppose the plates are large relative to the separation, so that edge effects can be neglected. Then , , and (the speed of electrons) are all functions of alone.
- Find , and as a functions of .
- Show that , and find the constant . This equation is called the Child–Langmuir law. It holds for other geometries as well, whenever space-charge limits the current. Notice that the space-charge limited diode does not obey Ohm’s law!
If you need hints, you will find the detailed steps to reach the solution in the book by Griffihts, problem 2.48.